Systems Neuroscience Imaging Resource
The Systems Neuroscience Imaging Resource (SNIR) makes tools for contemporary systems-level molecular anatomy accessible to NIMH intramural investigators. Current approaches to the investigation of brain circuits and systems require analyses of neuronal projections, gene expression, and protein distribution patterns at cellular or sub-cellular resolution across multiple brain regions. The SNIR provides access to appropriate hardware, software, wet lab procedures, training, support and expertise. The SNIR facilitates access to technologies such as high-throughput wide-field microscopy, deep tissue imaging via laser scanning confocal microscopy, and light sheet microscopy. It also facilitates the application of recently developed genetic, molecular, and imaging and image analysis techniques to the projects and problems of intramural investigators. A particular focus is the facilitation of work incorporating advances in 3D reconstruction of specified circuits, cell types, and protein distributions, combining modern clearing, image acquisition, and volume reconstruction methods.
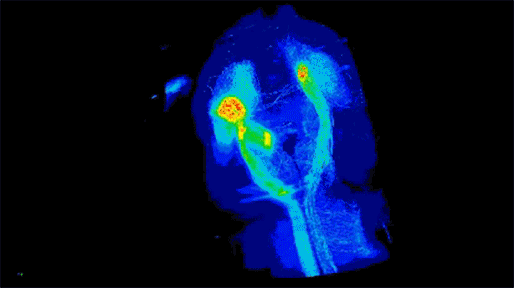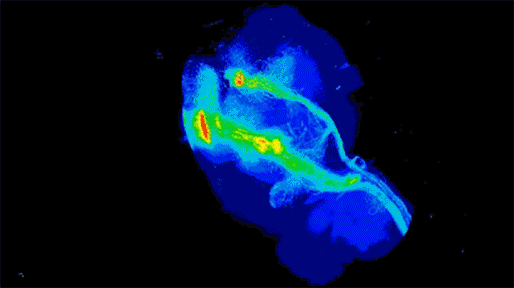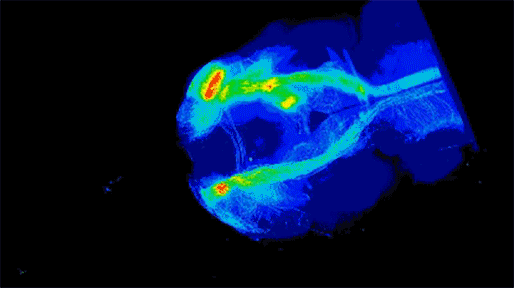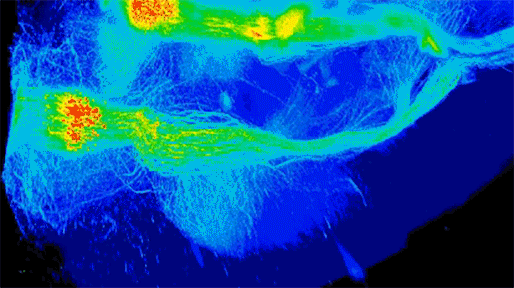
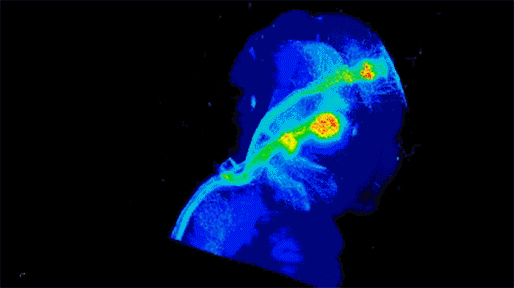
Neuronal projections from dopamine receptor expressing neurons in the mouse brain. Injections of an AAV with cre-dependent expression of the fluorescent reporter tdTomato were targeted bilaterally to nucleus accumbens of an adult mouse (performed by Juan Enriquez Traba while in Dr. Hugo Tejeda’s lab). Whole mouse brain was cleared using detergent-based protocols and imaged on a LaVision light-sheet microscope (performed by Ted Usdin). Background subtraction was performed with custom code for high-throughput processing (written by Snehashis Roy). Image pseudocoloring based on relative intensity and 3D visualization was performed in Arivis Vision4D software.”

