Archived Content
The National Institute of Mental Health archives materials that are over 4 years old and no longer being updated. The content on this page is provided for historical reference purposes only and may not reflect current knowledge or information.
Autism Blurs Brain Tissue’s Distinctiveness
Erodes Molecular Identities In Cortex – NIH-funded Study
• Press Release
Autism blurs the molecular differences that normally distinguish different brain regions, a new study suggests. Among more than 500 genes that are normally expressed at significantly different levels in the front versus the lower middle part of the brain's outer mantle, or cortex, only 8 showed such differences in brains of people with autism, say researchers funded, in part, by the National Institutes of Health.
"Such blurring of normally differentiated brain tissue suggests strikingly less specialization across these brain areas in people with autism" explained Daniel Geschwind, M.D., Ph.D., of the University of California Los Angeles, a grantee of the NIH's National Institute of Mental Health. "It likely reflects a defect in the pattern of early brain development."
He and his colleagues publish their study in post-mortem brains of people with the disorder online May 26, 2011 in the journal Nature.
In early development, different mixes of genes turn on in different parts of the brain to create distinct tissues that perform specialized functions. The new study suggests that the pattern regulating this gene expression goes awry in the cortex in autism, impairing key brain functions.
"This study provides the first evidence of a common signature for the seemingly disparate molecular abnormalities seen in autism," said NIMH director Thomas R. Insel, M.D. "It also points to a pathway-based framework for understanding causes of other brain disorders stemming from similar molecular roots, such as schizophrenia and ADHD."
In an earlier study, the researchers showed that genes that turn on and off together at the same time hold clues to the brain's molecular instructions. These "modules" of co-expressed genes can reveal genetic co-conspirators in human illness, through what Geschwind and colleagues call "guilt by association." A gene is suspect if its expression waxes and wanes in-sync with others in an illness-linked module.
Using this strategy, the researchers first looked for gene expression abnormalities in brain areas implicated in autism — genes expressed at levels different than in brains of healthy people. They found 444 such differently expressed genes in the cortex of postmortem brains of people with autism, compared to only 2 in the cerebellum. So they focused on the cortex.
Most of the same genes turned out to be differently expressed in the frontal cortex as in the temporal cortex (lower middle) of autistic brains. Of these, genes involved in synapses, the connections between neurons, tended to be under-expressed, when compared with healthy brains. Genes involved in immune and inflammatory responses tended to be over-expressed. Significantly, the same pattern held in a separate sample of brains.
In both autistic and healthy control brains, modules of co-expressed genes related to specific cell types and biological functions. Yet, none of the modules of co-expressed genes in autistic brains showed any of the differences in expression levels normally seen between the frontal and temporal cortex. This suggested that the normal molecular distinctions — the tissue differences — between these regions are nearly erased in the brain disorder. Strikingly, among 174 genes expressed at different levels between the two regions in two healthy control brains, none were expressed at different levels in brains of people with autism.
An analysis of gene networks revealed two key modules of co-expressed genes highly correlated with autism.
One module was made up of genes in a brain pathway involved in neuron and synapse development, which were under-expressed in autism. Many of these genes were also implicated in autism in previous, genome-wide studies. So several different lines of evidence now converge, pointing to genes in this M12 module (see picture below) as genetic causes of autism.
A second module of co-expressed genes (called M16), related to astrocytes and glial cells, was over-expressed in autism. These were determined not to be genetic causes of the illness, but likely related to secondary inflammatory, immune, or possible environmental factors involved in autism.
This newfound ability to see genes in the context of their positions in these modules, or pathways, provides hints about how they might work to produce illness, according to Geschwind and colleagues.
Follow-up studies should explore whether the observed abnormalities in the patterning of gene expression might also extend to other parts of the brain in autism, say the researchers.
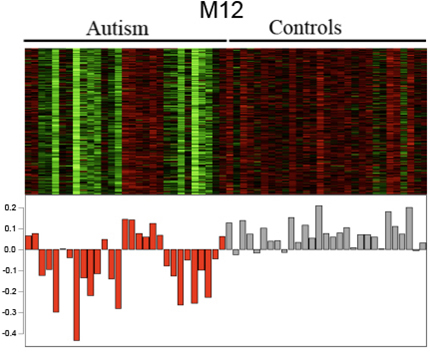
A module of co-expressed genes that code for neurons and their connections tend to be under-expressed in many individuals with autism (red), compared to controls (gray).
Reference
Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor R, Blencowe BJ, Geschwind DH. Transcriptomic Analysis of Autistic Brain Reveals Convergent Molecular Pathology. Nature. 2011 June 15.
About the National Institute of Mental Health (NIMH): The mission of the NIMH is to transform the understanding and treatment of mental illnesses through basic and clinical research, paving the way for prevention, recovery and cure. For more information, visit the NIMH website.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit the NIH website .
NIH…Turning Discovery Into Health®
