Archived Content
The National Institute of Mental Health archives materials that are over 4 years old and no longer being updated. The content on this page is provided for historical reference purposes only and may not reflect current knowledge or information.
Suspect Gene Variants Boost PTSD Risk after Mass Shooting
Profile of Risk Emerging for Trauma-triggered Molecular Scars
• Science Update
College students exposed to a mass shooting were 20-30 percent more likely to later develop post traumatic stress disorder (PTSD) symptoms if they harbored a risk version of a gene, NIMH-funded researchers have discovered. This boost in risk, traced to common variants of the gene that controls recycling of serotonin, was comparable to the risk conferred by close proximity to the shooting – for example, being in the room with the shooter versus just being on campus.
The discovery is the latest of several recently reported that collectively profile heightened biological vulnerability to developing PTSD following trauma – and the molecular scars it leaves in the brain.
For example, early this year, researchers linked high levels of a stress-triggered, estrogen-related hormone to PTSD symptoms in women, with certain versions of the hormone receptor’s gene conferring higher risk. A PET scan study in September traced increased PTSD symptoms to heightened levels of a serotonin receptor. Both studies suggest potential new drug targets for treating the disorder. Evidence is also mounting that trauma – particularly if experienced very early in life – can adversely alter the set-points of gene expression in brain stress circuits and compromise immune and inflammatory system function.
Gene-by-environment – caught in the act
By chance, researchers at Northern Illinois University (NIU) had already collected data on students’ PTSD symptoms prior to the 2008 murder-suicide that killed six on the Dekalb, Illinois campus.*
“This provided a rare opportunity to pinpoint not just a correlation but a cause – to document that such a tragedy can conspire with a risk gene to produce the disorder,” explained Kerry Ressler, M.D., Ph.D., of Emory University.
NIMH grantees Ressler, NIU’s Holly Orcutt, Ph.D., and colleagues, report on discovery of this gene-by-environment interaction online September 5th 2011 in the Archives of General Psychiatry.
Previous efforts to confirm such an interaction in PTSD had been confounded by lack of data on individuals’ pre-trauma symptoms. Any pre-existing symptoms must be taken into account to establish a common baseline – so that new symptoms that develop can confidently be pegged to the traumatic event.
By chance, before the tragedy, Orcutt’s team had prospectively surveyed PTSD symptoms in more than a thousand NIU undergraduate women, as part of a longitudinal study on predictors of sexual victimization, which can trigger the disorder. Within a few weeks after the tragedy, they seized the opportunity and – with help from a NIMH RAPID grant – conducted follow-up surveys, using the same measures, in subsets of the original sample – and then again after several months – to track symptom changes. Ressler’s team ultimately analyzed saliva samples from 235 women for gene type.
Previous studies had linked PTSD to a version of the gene that codes for the serotonin transporter (SERT), the protein on neurons that recycles the chemical messenger serotonin back into the cell after it is secreted into the synapse. So the researchers focused their genetic analysis on this variation, noting that it is “the most commonly described polymorphism in the psychiatric genetics literature.”
For example, this same site of genetic variation has also been linked to increased risk for anxiety - and, in some studies, increased risk for depression following stressful life events, although the latter findings remain controversial. Some hypothesize that these implicated variants may have less to do with conferring disease risk, per se, than with increased sensitivity to environmental influences more generally.**
Antidepressant medications, serotonin selective reuptake inhibitors (SSRIs), work by blocking SERT, thereby enhancing serotonin activity. SSRIs are the main medication treatment for PTSD.
Everyone inherits two copies of the SERT gene, one from each parent. So people can inherit one or two copies of risk-associated versions that are common in the population. Carrying any combination of these risk versions had been associated with increased risk for PTSD in 8 out of 9 previous studies.
The new study more definitively connects the dots between the environmental trigger and these risk gene types. Among 204 women without prior symptoms, 20 percent of those who showed acute symptoms within a few weeks after the shooting had developed PTSD symptoms when surveyed several months later. Proximity to the shooting and the risk gene types were about equally predictive of increased risk among this group.
These results come at a time of ferment in the field over confidence in gene-by-environment findings. A recent analysis by NIMH grantees of more than 100 such studies over the past decade uncovered what they call “publication bias.” They found that positive new findings were more likely to get published, while direct replications – which tend be less likely to confirm positive new findings – were under-reported. The net effect: an unintentional bias toward false positive reports. Notably, the researchers singled out as a prime example of this bias the scientific literature on serotonin transporter gene-by-environment interactions.
“How we measure environment may be at least as important as how we measure genetics, but to date, little effort has been focused on that,” noted Ressler. “We think that performing prospective studies in populations with shared trauma may be one way to 'hold constant' the environment variable, thus allowing for more clarity in the role of genetics.”
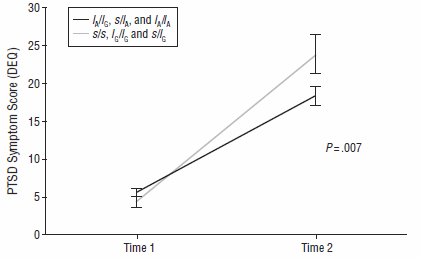
PTSD symptoms of NIU undergraduate women with a risk-associated serotonin transporter gene type (s/s, lG/lG, s/lG) increased 20-30 percent more than in classmates with a protective gene type after the 2008 campus shootings (Time 2). The increased risk was comparable to that conferred by close proximity to the shooting.
Source: Kerry Ressler, M.D., Ph.D., Emory University
Why Women are More Vulnerable
Earlier this year, Ressler and colleagues reported findings that may help to explain why women are twice as likely as men to develop PTSD. They linked PTSD symptoms in women to higher blood levels of what has been dubbed the “master regulator” of the stress response , a hormone called PACAP (pituitary adenyl cyclase-activating peptide).
PTSD symptoms were 5-fold higher in women with above average PACAP levels, compared to women with below average levels. Also in women only, a certain version of the gene that codes for PACAP’s receptor, PAC-1, conferred increased vulnerability. Experiments in rodents confirmed that this variable part of the PAC-1 gene is regulated, in part, by the female hormone estrogen.
This suggests that heightened vulnerability to PTSD in females may be traceable to this brain system critical to proper stress circuit function. Genetic variation in a different pathway may similarly be linked to increased risk for PTSD in men, say the researchers.
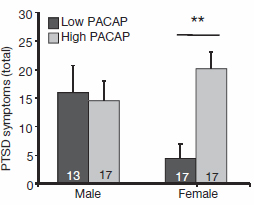
Females with higher-than-average levels of the stress-managing hormone PACAP had 5 times more PTSD symptoms than females with lower-than-average levels. By contrast, PACAP levels were unrelated to PTSD symptoms in males. Since the PACAP system is shaped, in part, by the female hormone estrogen, these differences could help to explain why women are twice as likely as men to develop the disorder.
Source: Kerry Ressler, M.D., Ph.D., Emory University
PACAP, “master regulator” of the stress response.
Molecular scars
The Emory researchers also found increased methylation – epigenetic regulation of gene expression in response to the environment – in the part of Pac1 associated with PTSD in both women and men. Adverse experiences can induce molecules called methyl groups to attach to DNA and block genes from turning on. This results in enduring changes in the proteins the genes express. These molecular scars can weaken the brain’s defenses against PTSD.
Indeed, methylation increases pervasively in PTSD, according to Ressler and colleagues. Notably, they pinpointed such increases in several genes implicated in inflammatory and immune system abnormalities that go along with PTSD. They also saw abnormalities in immune system chemical messengers, called cytokines. Increased blood levels of one such cytokine TNF-alpha, known to trigger stress response symptoms, correlated with a history of child abuse and cumulative life stresses.
Early trauma may deplete resilience molecule
In September, a NIMH-funded brain imaging study reported that levels of a type of serotonin receptor (1B) were markedly lower in stress circuits of PTSD patients than in others exposed to trauma. This protein on neurons, to which the neurotransmitter binds, plays a pivotal role in stress resilience and antidepressant effect. By contrast, PET scans revealed that people who had experienced trauma but didn’t develop PTSD had only slightly fewer receptors than healthy controls.
NIMH grantee Alexander Neumeister, M.D., of Mount Sinai School of Medicine, and colleagues, traced both the severity of symptoms and the depleted receptors largely to the age at which trauma was first experienced. The earlier the age and the more subsequent trauma exposures, the fewer receptors expressed and the more severe the PTSD symptoms and overlap with depression. The dearth of receptors likely reflects such features of patients’ trauma histories, with those who develop PTSD also having other genetic or environmental vulnerabilities, say the researchers.
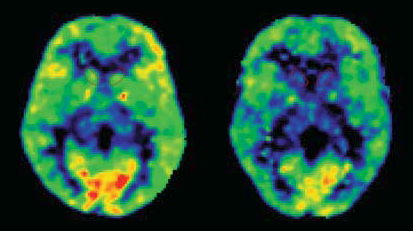
Patients with PTSD (right) had significantly fewer serotonin 1B receptors (yellow & red areas) in their brain stress circuits than healthy controls (left). PET scan images show destinations of a radioactive tracer that binds to serotonin 1B receptors. Front of brain is at bottom.
Source: Alexander Neumeister, M.D., Mount Sinai School of Medicine
Possible Uses: Risk profile and treatment targets?
Such epigenetic and genetic signatures of PTSD proneness in blood and brain, together with behavioral measures, may collectively prove useful in profiling a patient’s risk for developing the disorder. Molecules such as PACAP and the serotonin 1B receptor may also hold promise as potential targets of new drugs aimed at correcting specific abnormalities in the affected brain pathways, suggest the researchers.
NIMH RAPID grant helps salvage science from tragedy
- 2/14/08 Mass shooting on NIU campus
- 2/19/08 NIU researcher Holly Orcutt, Ph.D., contacts NIMH to discuss how to make the most of her prospectively collected data on PTSD and other parameters to learn from the tragedy.
- 3/26/08 Orcutt submits a concept for a follow-up study under the NIMH Rapid Assessment Post Impact of Disaster (RAPID) research program announcement – a unique time sensitive mechanism for expediting funding of research grants in response to emergency situations.
- 5/17/08 Grant application submitted.
- 6/18/08 Application undergoes peer review.
- 9/18/08 Grant awarded to NIU and Orcutt.
References
Acute and Posttraumatic Stress Symptoms in a Prospective Gene x Environment Study of a University Campus Shooting. Mercer KB, Orcutt HK, Quinn JF, Fitzgerald CA, Conneely KN, Barfield RT, Gillespie CF, Ressler KJ. Arch Gen Psychiatry. 2011 Sep 5. [Epub ahead of print]
PMID:21893641
A Critical Review of the First 10 Years of Candidate Gene-by-Environment Interaction Research in Psychiatry. Duncan LE, Keller MC. Am J Psychiatry. 2011 Sep 2. [Epub ahead of print]
PMID: 21890791
Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor.
Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V.Nature. 2011 Feb 24;470(7335):492-7. Erratum in: Nature. 2011 Sep 1;477(7362):120.
PMID:21350482
PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response.
Stroth N, Holighaus Y, Ait-Ali D, Eiden LE. Ann N Y Acad Sci. 2011 Mar;1220:49-59. doi: 10.1111/j.1749-6632.2011.05904.x. Review. PMID:21388403
The Effect of Early Trauma Exposure on Serotonin Type 1B Receptor Expression Revealed by Reduced Selective Radioligand Binding. Murrough JW, Czermak C, Henry S, Nabulsi N, Gallezot JD, Gueorguieva R, Planeta-Wilson B, Krystal JH, Neumaier JF, Huang Y, Ding YS, Carson RE, Neumeister A. Arch Gen Psychiatry. 2011 Sep;68(9):892-900. PMID:21893657
Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. Am J Med Genet B Neuropsychiatr Genet. 2011 Sep;156(6):700-8. doi: 10.1002/ajmg.b.31212. Epub 2011 Jun 28. PMID:21714072
Psychiatry: A molecular shield from trauma. Stein MB. Nature. 2011 Feb 24;470(7335):468-9. No abstract available. PMID:21350472
*http://en.wikipedia.org/wiki/Northern_Illinois_University_shooting
** http://www.theatlantic.com/magazine/archive/2009/12/the-science-of-success/7761/
